Persistent musculoskeletal pain is a global burden, second only to cardiovascular disease (Hegarty, 2018). Chronic or persistent pain is described as lasting three months, while also recognising it is multifactorial, complex and involves disease of the nervous system (Perrot et al., 2019). Persistent pain is linked to central sensitisation, describing changes to the central nervous system due to pain hypersensitivity (Woolf, 2011). Given the correlation between pain hypersensitivity and persistent pain, it seems very important to assess this sensitivity.
Many factors can influence pain hypersensitivity, including sustained peripheral nociception (inflammation or trauma), genetics, environmental and psychological factors. Certain cognitive factors, such as kinesiophobia and catastrophising, have been shown to influence pain sensitivity and are linked with persistent pain (Georgopoulos et al., 2019; Traxler, Hanssen, Lautenbacher, Ottawa, & Peters, 2019; Zusman, 2002). Pain hypersensitivity is considered an underlying contributing mechanism in pain becoming persistent, and indicates a poorer recovery (Scott, Jull, & Sterling, 2005; Sterling, Jull, Vicenzino, & Kenardy, 2003).
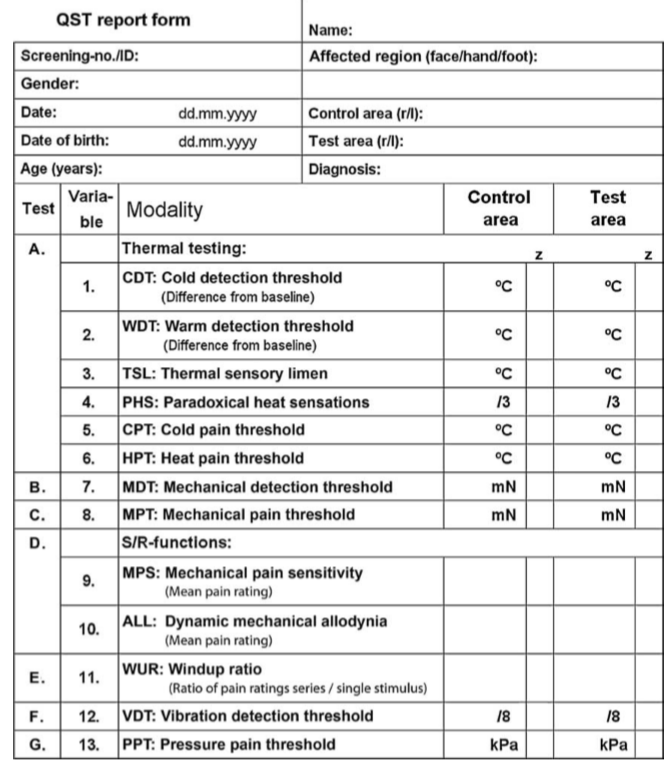
WHAT IS QST?
Quantitative sensory testing (QST) is an objective method of testing this pain hypersensitivity, where various stimuli are applied to the skin, ranging from vibration, light touch, proprioception and temperature, using standardised testing procedures and validated equipment (Georgopoulos et al., 2019). The recorded response is based on the patient’s reaction to the stimulus, providing an objective measure of their sensitivity to the stimulus, which may be influenced by cognitive factors (Hall, Briffa, Schafer, Tampin, & Moloney, 2015). It is not a measure of pain or sensation, but a measure of sensitivity (Hall et al., 2015).
The DFNS (German Research Network on Neuropathic Pain) developed a battery of tests, which are validated in assessing the somatosensory system (Rolke et al., 2006).
The battery includes:
Detection Thresholds
Warm Detection Threshold (WDT) & Cold Detection Threshold (CDT)
Touch (MDT - Mechanical Detection Threshold) & Vibration Detection Threshold (VDT)
Pain Thresholds
Cold Pain Threshold (CDT) & Heat Pain Threshold (HPT)
Pressure (PPT – Pressure Pain Threshold) & Pinprick (MPT – Mechanical Pain Threshold)
Temporal Summation
assessing an increasing response to sustained or subsequent stimuli
THERMAL DETECTION
A thermal sensory testing device is used, placing a thermode on the skin being tested. The temperature is increased or decreased in regular increments from 32˚C, with the patient pressing a button when they detect a change in temperature (Hall et al., 2015).
This is repeated with pain thresholds, where the patient presses the button when the temperature becomes ‘painful’ to the patient (Rolke, Baron, et al., 2006).
Reduced detection of temperature changes can indicate reduced small nerve fibre function, with evidence of reduced detection in cervical and lumbar radiculopathies on their affected side (Tampin, Slater, & Briffa, 2013; Tampin, Slater, Hall, Lee, & Briffa, 2012). This could suggest reduced nerve conductivity, however other studies have found bilateral reduction in detection following a unilateral lesion, possibly suggesting central plasticity changes (Tampin, Slater, et al., 2013; Tampin, Vollert, & Schmid, 2018).
Increased sensitivity to heat has been highlighted in many musculoskeletal conditions, and suggests peripheral sensitisation mechanisms (Hall et al., 2015). Sensitivity to cold commonly occurs secondary to peripheral nerve injury, but is not strongly correlated with nerve damage or a patient’s reported pain levels (Blumenstiel et al., 2011). Cold hyperalgesia was found to moderately predict poor recovery and long-term disability following whiplash-associated disorders, suggesting possibly central and peripheral mechanisms occurring (Goldsmith, Wright, Bell, & Rushton, 2012).
MECHANICAL DETECTION
Mechanical detection thresholds (MDT) are measured using standardised von Frey filaments, which exert force on the skin from 0.25mN to 512 mN (Rolke, Baron, et al., 2006). The filament bends upon touching the skin, without piercing or activating nociceptor fibres. Incremental filaments are used, to determine the point at which the individual detects light touch (Hall et al., 2015). Five tests were performed, with the average of the tests being the resultant threshold.
Reduced mechanical detection (reduced ability to detect light touch) may indicate a peripheral nerve lesion, if the loss occurs in a dermatomal or cutaneous distribution (Hall et al., 2015). A heightened response to mechanical stimuli, termed mechanical hyperalgesia, has been reported in those with fibromyalgia, knee osteoarthritis and musculoskeletal pain (Fingleton, Smart, Moloney, Fullen, & Doody, 2015; Scott et al., 2005; Tampin, Briffa, & Slater, 2013).
Mechanical pain thresholds (MPT) are measured using weighted pinprick stimuli, exerting forces at 8, 16, 32, 64, 128, 256 and 512 mN (Rolke, Baron, et al., 2006, p. 233), applied at 2 seconds on then 2 seconds off incrementally until ‘sharpness’ is detected. Reduced detection ability may indicate neuropathy, with hyperalgesia suggesting peripheral and central sensitisation (Georgopoulos et al., 2019).
TEMPORAL SUMMATION
The same pinprick equipment from mechanical testing is used, with the same pinprick stimulus (128mN used for facial testing, 256mN used for peripheral areas) now being applied for 10 repetitions, held for 1 second over the same 1cm² area (Rolke, Baron, et al., 2006). The individual provides a ‘pain rating’ for a single pinprick and a rating for the 10 repetitions, using a 0-100 numerical rating scale (Rolke, Baron, et al., 2006). This is repeated 5 times, with the mean pain rating used. Temporal summation or wind-up measures changes in excitability in spinal cord neurons, with repeated repetitions of a stimulus. The increase should plateau after 5 repetitions, as the input stimulates a central response. In those with central changes, their pain rating may continue to increase with repetition (Hall et al., 2015; Woolf, 2011).
VIBRATION THRESHOLD
A Rydel-Seiffer tuning fork (64 Hz) is placed over a bony protuberance (ulna styloid, medial malleolus, mandible), with the subject reporting once vibration is no longer felt (Rolke, Magerl, et al., 2006). This was repeated three times, with the mean calculated.
A reduction in vibration detection can indicate peripheral nerve damage in diabetic neuropathy, peripheral nerve lesions, radiculopathies and those with neuropathic pain (Schmid, Soon, Wasner, & Coppieters, 2012; Tampin et al., 2018). However, widespread changes in vibration detection have also been reported in this population, suggesting central processing mechanisms are also occurring. A reduction in vibration detection have been seen in non-neuropathic pain populations, again indicating central mechanisms may be involved (Fingleton et al., 2015; Tampin et al., 2018; Tucker et al., 2007).
PRESSURE PAIN THRESHOLD
The point at which pressure becomes painful is tested using a pressure algometer. The probe is placed over the muscle belly (masseter, thenar muscles), with pressure increasing until the patient reports ‘pain’ (Rolke, Magerl, et al., 2006). This can detect hyperalgesia (increase in function) or hypoaesthesia (loss of function). Increased sensitivity to pressure at local and distal regions was found in those suffering from whiplash-associated disorders, cervical radiculopathy and non-specific arm pain (Pedler, Kamper, & Sterling, 2016). It was also detected in those with knee osteoarthritis, resulting in poorer post-operative outcomes and poorer response to exercise programs (Fingleton et al., 2015).
DYNAMIC TESTING
The above tests provide a static understanding of an individual’s somatosensory system, however dynamic testing can also be completed (Treede, 2019). Dynamic tests look at how a person’s system adapts to subsequent stimuli, initially subjecting the person to a painful stimulus (such as immersing a hand into icy water), then retesting another threshold (such as pressure pain threshold at a distant site). The icy water will alter the person’s response to pressure pain threshold testing, either being inhibitory or facilitatory, which is called conditioned pain modulation (Treede, 2019).
Conditioned pain modulation (CPM) involves descending control from the brainstem, with central processes which are consistent with depression and other psychological disorders, even when no nociceptor driver can be found (Georgopoulos et al., 2019). It suggests that CPM is involved with psychological aspects contributing to persistent pain states (Georgopoulos et al., 2019).
WHY IS IT RELEVANT TO PHYSIO?
Patients with centrally driven pain tend to respond more favourably to education, graded exercise and a multidisciplinary approach rather than a nociceptive, peripheral approach (Fersum, O'Sullivan, Skouen, Smith, & Kvale, 2013; Oliveira et al., 2018). Detecting this hypersensitivity is challenging, with some self-report questionnaires highlighting risk factors associated with persistent pain. Unfortunately, these factors are also seen in those without pain, or those with predominantly nociceptive mechanisms (Georgopoulos et al., 2019).
A systematic review by Georgopoulos (2019) found pain hypersensitivity predicted those with a poorer prognosis and delayed recovery. Poor outcomes were independent of diagnosis, with hypersensitivity predicting a poor prognosis across multiple injuries, to both spinal and peripheral musculoskeletal areas. Quantitative sensory testing may help differentiate those with a poorer prognosis, who would likely benefit from treatment focusing on function and disability, rather than pain. It is also predictive in identifying those with higher postoperative pain levels, and poorer post-surgical recovery.
IN THE CLINIC?
While QST provides an effective measure of prognosis, not many of us have access to the equipment required. There are clinical bedside tests which are routinely used, to attempt to replicate the tests completed in laboratories.
Zhu and colleagues (2019) compared the current clinical battery of tests to the previously mentioned QST battery. They found six tests had moderate to high correlation with the QST equivalent. The tests highlighted in blue indicate the tests with the strongest clinical correlation to the research methods.
All tests were completed by testing an unaffected, symptom-free area first, followed by testing of the symptomatic area. This could be the contralateral limb or a proximal region to their symptoms, if the contralateral limb was unavailable (e.g. bilateral symptoms). Tests were completed once at the unaffected area, then once at the affected site.
The patient was asked if the sensation was increased, decreased or the same as the ‘test’ site. They were then asked for a pain rating on the numerical rating scale, with 0/10 being pain-free and 10/10 being the worst pain possible.

Zhu et al, 2019 page 1830. Cold Detection Threshold (CDT), Warm Detection Threshold (WDT) & Mechanical Detection Threshold with cotton wool (MDT-cotton).
The Cold Detection Threshold (CDT) and Warm Detection Threshold (WDT) involved holding a 50c coin (in Australian currency) or a 50p coin (UK currency) on the patient’s skin. The cold coin was kept at room temperature while the warm coin was held in the therapist’s pocket for 30 minutes, prior to testing. The patient compared the temperature to the unaffected region.
The Mechanical Detection Threshold with cotton wool (MDT-cotton) involved lightly stroking the area with cotton wool, ensuring minimal pressure is placed on the ball. This was again compared to the symptom-free site.

Zhu et al, 2019 page 1830. Cold Pain Threshold (CPT).
Cold Pain Threshold (CPT) testing was completed using an ice cube placed inside a plastic bag. The bag was held on the skin for 10 seconds, initially at a symptom-free site then at the affected area. The patient was again asked if sensation was increased, decreased or same as the test site, then asked for a pain rating.

Zhu et al, 2019 page 1830. Pressure Pain Threshold with eraser (PPT - eraser), Pressure Pain Threshold with thumb (PPT - thumb).
Pressure Pain Thresholds (PPT) were measured using an eraser on the end of a pencil and the therapist’s thumb.
Both tests involved holding the eraser or thumb on the patient’s skin for 10 seconds, applying enough pressure to create an indentation and some whitening/blanching of the skin. The unaffected area was tested first, repeated at the affected site. The patient again reported an increase, decrease or same sensation, and a pain rating scale, compared to the unaffected area.
While other tests are also commonly used, especially in determining sensation, these tests are the most clinically relevant in testing pain hypersensitivity. Sharp/blunt sensation using a toothpick, and light touch using a tissue, are still valid in assessing sensation loss, however the above tests focus on pain hypersensitivity, as an indicator of central sensitisation.
Quantitative sensory testing is useful in determining those with a poor prognosis, by identifying those with features of central sensitisation. This can alter our treatment approach, by reducing hands-on therapy and focusing on addressing kinesiophobia, depression, anxiety and catastrophising, which can perpetuate central mechanisms. Clinically, we can utilise some simple tests which are correlated to those used in research, to help identify those at risk of developing persistent pain, and altering our treatment approach when required.
Alicia Rayner
this post is reposted with permission from Rayner and Smale's blog.
REFERENCES
Blumenstiel, K., Gerhardt, A., Rolke, R., Bieber, C., Tesarz, J., Friederich, H. C., . . . Treede, R. D. (2011). Quantitative Sensory Testing Profiles in Chronic Back Pain Are Distinct From Those in Fibromyalgia. Clinical Journal of Pain, 27(8), 682-690.
Fersum, K. V., O'Sullivan, P., Skouen, J. S., Smith, A., & Kvale, A. (2013). Efficacy of classification-based cognitive functional therapy in patients with non-specific chronic low back pain: A randomized controlled trial. European Journal of Pain, 17(6), 916-928.
Fingleton, C., Smart, K., Moloney, N., Fullen, B. M., & Doody, C. (2015). Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis and Cartilage, 23(7), 1043-1056.
Georgopoulos, V., Akin-Akinyosoye, K., Zhang, W. Y., McWilliams, D. F., Hendrick, P., & Walsh, D. A. (2019). Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: a systematic review and meta-analysis. Pain, 160(9), 1920-1932.
Goldsmith, R., Wright, C., Bell, S. F., & Rushton, A. (2012). Cold hyperalgesia as a prognostic factor in whiplash associated disorders: A systematic review. Manual Therapy, 17(5), 402-410.
Hall, T., Briffa, K., Schafer, A., Tampin, B., & Moloney, N. (2015). Quantitative Sensory Testing: implications for clinical practice. In G. Jull, A. Moore, D. Falla, J. Lewis, C. McCarthy, & M. Sterling (Eds.), Grieve's modern musculoskeletal physiotherapy: vertebral colum and peripheral joints (pp. 194-201). United Kingdom: Elsevier Health Sciences.
Hegarty, D. (2018). Abstracts from Proceedings Cork CRPS 2017: IASP Special Interest Group in Complex Regional Pain Syndrome (CRPS). Pain reports, 3(1), e635-e635.
Oliveira, C. B., Maher, C. G., Pinto, R. Z., Traeger, A. C., Lin, C. W. C., Chenot, J. F., . . . Koes, B. W. (2018). Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. European Spine Journal, 27(11), 2791-2803.
Pedler, A., Kamper, S. J., & Sterling, M. (2016). Addition of posttraumatic stress and sensory hypersensitivity more accurately estimates disability and pain than fear avoidance measures alone after whiplash injury. Pain, 157(8), 1645-1654.
Perrot, S., Cohen, M., Barke, A., Korwisi, B., Rief, W., Treede, R.-D., . . . Chro, I. T. C. (2019). The IASP classification of chronic pain for ICD-11: chronic secondary musculoskeletal pain. Pain, 160(1), 77-82.
Rolke, R., Baron, R., Maier, C., Tolle, T. R., Treede, R. D., Beyer, A., . . . Wasserka, B. (2006). Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain, 123(3), 231-243.
Rolke, R., Magerl, W., Campbell, K. A., Schalber, C., Caspari, S., Birklein, F., & Treede, R. D. (2006). Quantitative sensory testing: a comprehensive protocol for clinical trials. European Journal of Pain, 10(1), 77-88.
Schmid, A. B., Soon, B. T. C., Wasner, G., & Coppieters, M. W. (2012). Can widespread hypersensitivity in carpal tunnel syndrome be substantiated if neck and arm pain are absent? European Journal of Pain, 16(2), 217-228.
Scott, D., Jull, G., & Sterling, M. (2005). Widespread sensory hypersensitivity is a feature of chronic whiplash-associated disorder but not chronic idiopathic neck pain. Clinical Journal of Pain, 21(2), 175-181.
Sterling, M., Jull, G., Vicenzino, B., & Kenardy, J. (2003). Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain, 104(3), 509-517.
Tampin, B., Briffa, N. K., & Slater, H. (2013). Self-reported sensory descriptors are associated with quantitative sensory testing parameters in patients with cervical radiculopathy, but not in patients with fibromyalgia. European Journal of Pain, 17(4), 621-633.
Tampin, B., Slater, H., & Briffa, N. K. (2013). Neuropathic Pain Components Are Common in Patients With Painful Cervical Radiculopathy, but Not in Patients With Nonspecific Neck-Arm Pain. Clinical Journal of Pain, 29(10), 846-856.
Tampin, B., Slater, H., Hall, T., Lee, G., & Briffa, N. K. (2012). Quantitative sensory testing somatosensory profiles in patients with cervical radiculopathy are distinct from those in patients with nonspecific neck-arm pain. Pain, 153(12), 2403-2414.
Tampin, B., Vollert, J., & Schmid, A. B. (2018). Sensory profiles are comparable in patients with distal and proximal entrapment neuropathies, while the pain experience differs. Current Medical Research and Opinion, 34(11), 1899-1906.
Traxler, J., Hanssen, M. M., Lautenbacher, S., Ottawa, F., & Peters, M. L. (2019). General versus pain-specific cognitions: Pain catastrophizing but not optimism influences conditioned pain modulation. European Journal of Pain, 23(1), 150-159.
Treede, R. D. (2019). The role of quantitative sensory testing in the prediction of chronic pain. Pain, 160, S66-S69.
Tucker, A. T., White, P. D., Kosek, E., Pearson, R. M., Henderson, M., Coldrick, A. R., . . . Kidd, B. L. (2007). Comparison of vibration perception thresholds in individuals with diffuse upper limb pain and carpal tunnel syndrome. Pain, 127(3), 263-269.
Woolf, C. J. (2011). Central sensitization: Implications for the diagnosis and treatment of pain. Pain, 152(3), S2-S15.
Zhu, G. C., Bottger, K., Slater, H., Cook, C., Farrell, S. F., Hailey, L., . . . Schmid, A. B. (2019). Concurrent validity of a low-cost and time-efficient clinical sensory test battery to evaluate somatosensory dysfunction. European Journal of Pain, 23(10), 1826-1838.
Zusman, M. (2002). Forebrain-mediated sensitization of central pain pathways: 'non-specific' pain and a new image for MT. Manual Therapy, 7(2), 80-88.
- NEW - Online Discussion Group
- Live cases
- webinars
- lecture
- Live Q&A
- over 600 videos - hundreds of techniques and more!
- Check out MMT Insiders















Post a Comment
Post a Comment